Abstract
ELANE-related severe congenital neutropenia (SCN) is a disorder wherein numerous heterozygous mutations in the ELANE gene lead to misfolding and mislocalization of mutant neutrophil elastase, resulting in the death of myeloid cells and block in neutrophil differentiation.
Currently, SCN is treated with daily injections of granulocyte colony-stimulating factor (G-CSF), but patients on this therapy are at risk of developing myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). The only other effective therapy is allogeneic hematopoietic stem cell transplantation that may involve graft versus host disease and risk of serious infections. Thus, there is great interest in safe and efficient therapeutic alternatives.
Emendo Biotherapeutics has developed a novel CRISPR-based (OMNI A1) ex-vivo gene editing strategy that involves specific excision of disease-causing ELANE mutant allele. This treatment was tested on CD34 + HSCs from SCN patients and resulted in a significant improvement in the maturation of myeloid cells and their differentiation to normal functional neutrophils.
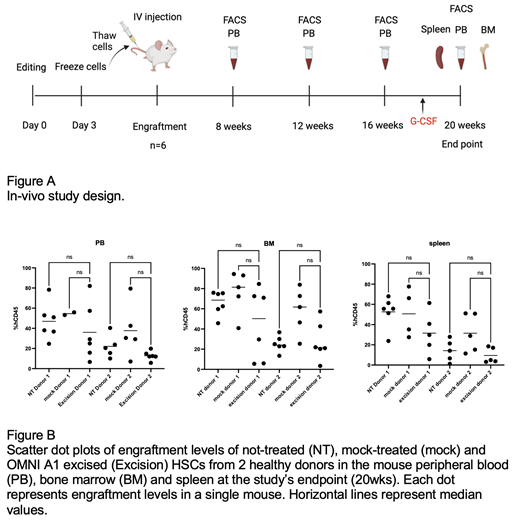
To assess the feasibility and safety of this editing strategy, we conducted an in vivo study using human HSCs derived from two healthy donors that were engrafted into a NOD scid gamma (NSG) immunocompromised mice model. The study involved a single iv injection of HSCs that were either not-treated (NT), electroporated without delivery of CRISPR nuclease (mock) or edited with Emendo's novel nuclease, OMNI A1,(excision). Each group consisted of 6 NSG female mice and an additional group of 4 mice served as vehicle control (no HSCs). All animals were subjected to total body irradiation, 1 day prior to the treatment, at a dose of 250 cGy. Animals were subjected to retro-orbital blood collection, at 4 designated time points during the study (Figure A), for CD45 + cell counts using FACS. All animals were evaluated for systemic reactions, clinical signs and body weight changes during the study period. At the end of the study period, animals were subjected to peripheral blood (PB), bone marrow (BM) and spleen sampling. FACS analysis of CD45 + cells was performed on those samples to determine the engraftment efficiency. Furthermore, excision levels for ELANE were measured in the engrafted cells by droplet digital PCR (ddPCR). Finally, multilineage differentiation capacity of the engrafted cells was evaluated by FACS.
Analysis of human CD45 + cells, at 20 weeks, showed comparable levels of engraftment of the not-treated, mock treated and excised HSCs in the mouse PB, BM and spleen (No statistically significant difference, Kruskal Wallis test) (Figure B). Excised cells were detected in the mouse BM and excision levels in some of the mice were comparable to those measured in the HSCs prior to engraftment (about 30-36%). Human-derived cells from the BM and spleen of mice engrafted with excised HSCs showed similar multilineage differentiation capacity as obtained in mice engrafted with either NT or mock-treated HSCs. Engrafted human CD45 + cells differentiated into neutrophils (CD66b +), myeloid cells (CD33 +), Lymphocytes: B-cells (CD19 +) and T-cells (CD3 +). In addition, a population of engrafted hematopoietic CD34 + cells was detected in the BM. Excised HSCs of both donors gave rise to all lineages tested, as efficient as the NT and the mock groups. Finally, engrafted mice showed no systemic reactions, abnormal clinical signs or loss of body weight.
These data show that edited cells can be engrafted successfully, maintain their excision profile and populate the bone marrow. This study also supports the safety of the OMNI A1 therapeutic composition.
Povodovski: Emendo Biotherapeutic: Current Employment. Makaryan: Emendo Biotherapeutic: Research Funding. Sabo: Emendo Biotherapeutic: Research Funding. Dicken: Emendo Biotherapeutic: Ended employment in the past 24 months. Herman: Emendo Biotherapeutic: Current Employment. Emmanuel: Emendo Biotherapeutic: Current Employment. Dale: X4 Pharmaceuticals: Consultancy, Honoraria, Research Funding.